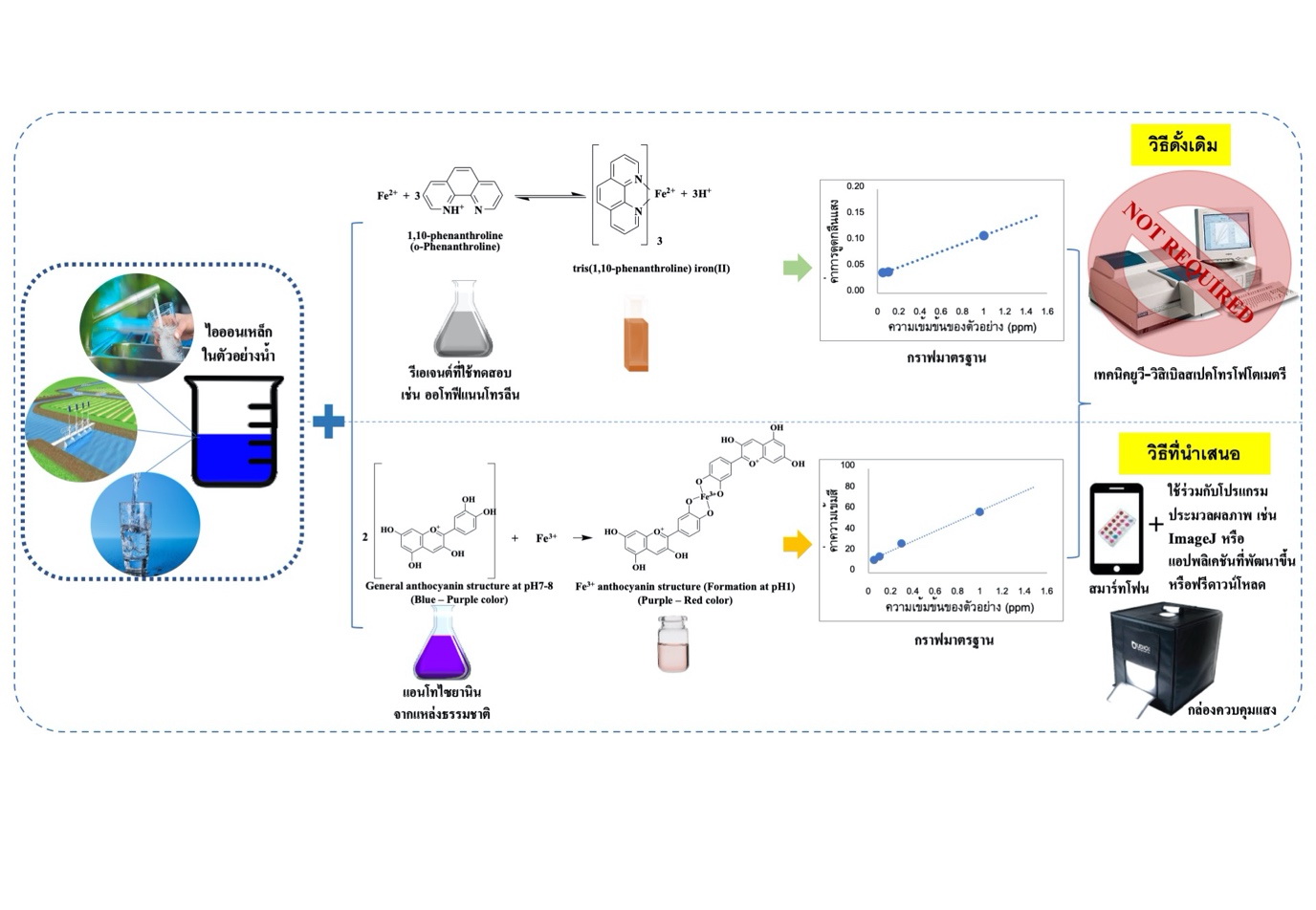
การใช้สมาร์ทโฟนเป็นอุปกรณ์ตรวจวัดทางสีสำหรับการหาปริมาณไอออนเหล็กในน้ำโดยใช้แอนโทไซยานินเป็นรีเอเจนต์
Main Article Content
Abstract
Waranphat Rattanakaroonjit, Rattapol Meelapsom, Saksri Supasorn and Purim Jarujamrus
รับบทความ: 24 มกราคม 2563; แก้ไขบทความ: 7 พฤษภาคม 2563; ยอมรับตีพิมพ์: 16 พฤษภาคม 2563
บทคัดย่อ
น้ำในธรรมชาติจะมีไอออนเหล็กละลายอยู่ เหล็กเป็นธาตุที่มีประโยชน์ต่อร่างกาย แต่หากเข้าสู่ร่างกายในปริมาณมากเกินไปจะก่อให้เกิดอันตรายต่อมนุษย์ได้ ดังนั้นหน่วยงานของภาครัฐที่เกี่ยวข้อง อาทิ องค์การอนามัยโลก รวมถึงกระทรวงสาธารณสุขและกรมทรัพยากรน้ำบาดาลได้กำหนดค่าความเข้มข้นมาตรฐาน (เกณฑ์กำหนดสูงสุด) เพื่อควบคุมปริมาณไอออนของเหล็กที่ปนเปื้อน การตรวจวิเคราะห์หาปริมาณไอออนเหล็กจึงมีความสำคัญ โดยวิธีการตรวจวิเคราะห์หาปริมาณไอออนเหล็กแบบมาตรฐานมีหลายวิธี เช่น เทคนิคทางสเปกโทรสโกปี และเทคนิคทางไฟฟ้าเคมีต่าง ๆ ซึ่งเทคนิคดังกล่าวมีสภาพไวโดยสามารถตรวจวัดได้ในระดับต่ำ ๆ และมีความจำเพาะเจาะจงสูง แต่มีข้อจำกัดคือยังต้องใช้เครื่องมือขั้นสูงที่มีราคาสูงและมีขั้นตอนที่ยุ่งยากที่ต้องอาศัยทักษะหรือผู้เชี่ยวชาญในการตรวจวิเคราะห์เชิงปริมาณ ปัจจุบันได้มีผู้วิจัยหลายกลุ่มมุ่งพัฒนาวิธีการวิเคราะห์เชิงปริมาณโดยใช้การวิเคราะห์ทางสีโดยการใช้สมาร์ทโฟนร่วมกับโปรแกรม ImageJ เพื่อลดข้อจำกัดด้านการใช้เครื่องมือขั้นสูงที่มีราคาสูงและลดขั้นตอนที่ยุ่งยากซับซ้อนโดยไม่ต้องอาศัยทักษะหรือผู้เชี่ยวชาญในการตรวจวิเคราะห์เชิงปริมาณ นอกจากนี้ยังใช้แอนโทไซยานินที่สกัดได้จากธรรมชาติเป็นรีเอเจนต์ทดแทนการใช้รีเอเจนต์ทางเคมีในการตรวจวิเคราะห์หาปริมาณไอออนเหล็กในน้ำตัวอย่างจริง ซึ่งในบทความวิจัยหลายบทความได้รายงานว่าการใช้สมาร์ทโฟนร่วมกับโปรแกรม ImageJ มีประสิทธิภาพเทียบเคียงกับวิธีมาตรฐาน และอาจสามารถประยุกต์ใช้สำหรับการทำปฏิบัติการการหาปริมาณโลหะหนักสำหรับวิชาเคมีระดับมัธยมศึกษาเพื่อเพิ่มโอกาสให้นักเรียนได้ลงมือทำปฏิบัติการอีกด้วย
คำสำคัญ: ไอออนเหล็ก แอนโทไซยานิน เคมีวิเคราะห์ สมาร์ทโฟน อุปกรณ์ตรวจวัดทางสี
Abstract
Iron ions can be found in water resources. Iron is a beneficial element for health, but excess iron ions are harmful to life. Therefore, involved government agencies, World Health Organization, Ministry of Public Health and Department of Groundwater Resources, have been stated the maximum acceptable concentration of contaminated iron ions for the water quality control. Conventional techniques such as spectroscopy and electrochemical techniques are utilized for routine analysis. Although, these techniques provide high sensitivity and selectivity for analysis, but still require expensive instruments and complicated procedure to operate advanced instruments along with an expert for quantitative analysis. Recently, an alternative method has been developed for quantitative analysis of iron ions in water using a smartphone coupled with ImageJ software as colorimetric analyzer. The developed method offers great potential with obvious advantages over the conventional techniques such as convenient, cost–effective, and suitable for unskilled user. In addition, anthocyanin extracted from natural sources was also used as the indicator instead of chemical reagents for the determination of iron ions. Moreover, smartphone–assisted colorimetric analysis of iron ions in water can provide reliable results that are comparable to those obtained from complicated laboratory advanced instruments and could possibly lead the development of hands on experiment for demonstrating students in high school.
Keywords: Iron ions, Anthocyanin, Analytical chemistry, Smartphone, Colorimetric analysis
Note: สามารถดาวน์โหลด Graphical Abstract ได้จาก Supplementary files
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
References
Barcelo, J. M. (2014). Detection of copper (II) and iron (III) in aqueous solutions using the spectroscopic characteristics of bugnay (Antidesma bunius). Annals of Tropical Research 36(1): 101–117.
Birch, N. C., and Stickle, D. F. (2003). Example of use of a desktop scanner for data acquisition in a colorimetric assay. Clinica Chimica Acta 333: 95–96.
Buchweitz, M., Brauch, J., Carle, R., and Kammerer, D. R. (2013a). Application of ferric anthocyanin chelates as natural blue food colorants in polysaccharide and gelatin based gels. Food Research International 51(1): 274–282.
Buchweitz, M., Brauch, J., Carle, R., and Kammerer, D. R. (2013b). Colour and stability assessment of blue ferric anthocyanin chelates in liquid pectin–stabilised model systems. Food Chemistry 138(2–3): 2026–2035.
Buchweitz, M., Carle, R., and Kammerer, D. R. (2012a). Bathochromic and stabilising effects of sugar beet pectin and an isolated pectic fraction on anthocyanins exhibiting pyrogallol and catechol moieties. Food Chemistry 135(4): 3010–3019.
Buchweitz, M., Nagel, A., Carle, R., and Kammerer, D. R. (2012b). Characterisation of sugar beet pectin fractions providing enhanced stability of anthocyanin–based natural blue food colourants. Food Chemistry 132(4): 1971–1979.
Burger, W., and Burge, M. J. (2009). Principles of Digital Image Processing: Fundamental Techniques. Berlin: Springer.
Byrne, L., Barker, J., Pennarun–Thomas, G., Diamond, D., and Edwards, S. (2000). Digital imaging as a detector for generic analytical measurements. TrAC Trends Analytical Chemistry 19: 517–522.
Cantrell, K., Erenas, M. M., Orbe–Payá, I. d. and Capitán-Vallvey L. F. (2010). Use of the hue parameter of the hue, saturation, value color space as a quantitative analytical parameter for bitonal optical sensors. Analytical Chemistry 82: 531–542.
Chaiyachok, V. (2009). Treatment of Heavy Metal Wastewater Using Green Liquor Dregs. Master thesis, M.S. (Engineering). Bangkok: Graduate School, Chulalongkorn University. (in Thai)
Chaiyo, S. (2012). Method Development for Trace Copper and Mercury Analysis Using Stripping Voltammetry. Master thesis, M.Sc. (Chemistry). Bangkok: Graduate School, Srinakharinwirot University. (in Thai)
Chandrasekhar, J., Madhusudhan, M. C., and Raghavarao, K. S. M. S. (2012). Extraction of anthocyanins from red cabbage and purification using adsorption. Food and Bioproducts Processing 90(4): 615– 623.
Cheng, G. W., and Crisosto, C. H. (1995). Browning potential, phenolic composition, and polyphenoloxidase activity of buffer extracts of peach and nectarine skin tissue. Journal of the American Society for Horticultural Science 120(5): 835–838.
Choodum, A., and Daeid, N. N. (2011). Rapid and semi–quantitative presumptive tests for opiate drugs. Talanta 86: 284–292.
Choodum, A., Kanatharana P., Wongniramaikul, W., and NicDaeid N. (2012). Rapid quantitative colourimetric tests for trinitrotoluene (TNT) in soil. Forensic Science International 222: 340–345.
Choodum, A., Kanatharana P., Wongniramai-kul, W., and NicDaeid N. (2013). Using the iPhone as a device for a rapid quantitative analysis of trinitrotoluene in soil. Talanta 115: 143–149.
da Costa, C. T., Nelson, B. C., Margolis, S. A., and Horton, D. (1998). Separation of blackcurrant anthocyanins by capillary zone electrophoresis. Journal of Chromatography A 799(1–2): 321-327.
Department of Groundwater Resources, (1999). Water Quality Standard. Retrieved from http://plan.dgr.go.th/school/5.pdf, December 4, 2019. (in Thai)
Epperson, P. M., Sweedler, J. V., Bilhorn, R. B., Sims, G. R., and Denton, M. B. (1988). High performance charge transfer device detectors. Analytical Chemistry 60: 327A– 335A.
Fakayode, S. O., King, A. G., Yakubu, M., Mohammed, A. K. and Pollard, D. A. (2012). Determination of Fe content of some food items by flame atomic absorption spectroscopy (FAAS): A guided–inquiry learning experience in instrumental analysis laboratory. Journal of Chemical Education 89(1): 109–113.
Ferreira, T., and Rasband, W. (2012). ImageJ User Guide IJ 1.46r. Bethesda, MD: National Institutes of Health.
Gao, L., and Mazza, G. (1994). Quantitation and distribution of simple and acylated anthocyanins and other phenolics in blueberries. Journal of Food Science 59(5): 1057–1059.
Gao, L., and Mazza, G. (1995). Characterization, quantitation, and distribution of anthocyanins and colorless phenolics in sweet cherries. Journal of Agricultural and Food Chemistry 43(2): 343–346.
Gil, M. I., Holcroft, D. M., and Kader, A. A. (1997). Changes in strawberry anthocya-nins and other polyphenols in response to carbon dioxide treatments. Journal of Agricultural and Food Chemistry 45(5): 1662–1667.
Giorgianni, E. J., and Madden, T. E. (1998). Digital Color Management: Encoding Solutions. United Kingdom: Wiley.
Giusti, M. M., and Wrolstad, R. E. (2001). Cha-racterization and measurement of anthocyanins by uv–visible spectroscopy. Current Protocols in Food Analytical Chem-istry 00(1): F1.2.1–F1.2.13.
Harris, D.C. (1995). Quantitative Chemical Analysis. 3rd ed. United States: Marcel Dekker.
Henretig, F. M., and Temple, A. R. (1984). Acute iron poisoning in Children. Emergency Medicine Clinics of North America 4: 575–586.
Hovinen, J., Lahti, M., and Vilpo, J. (1999). Spectrophotometric determination of thiocyanate in human saliva. Journal of Chemical Education 76(9): 1281.
Ibrahim, A. Q., Onyenekwe P. C., and Nwaedozic I. M. (2014). An efficiency assessment of lower usuma water treatment plant in Abuja. Metropolis, Nigeria. IOSR Jour-nal of Environmental Science, Toxicology and Food Technology [Online], 8, Ver.II. (pp. 46–53). Retrieved from http://www.scribd.com/document/250619321/, April 28, 2020.
Jarujamrus, P., Meelapsom, R., Pencharee, P., Obma, A., Amatatongchai, M., Ditcharoen, N., Chairam, S., and Tamuang, S., (2018). Use of a smartphone as a colorimetric analyzer in paper–based devices for sensitive and selective determination of mercury in water samples. Analytical Science 34: 75-81.
Jayawardane, B. M., McKelvie, I., and Kolev, S. (2012). A paper–based device for measurement of reactive phosphate in water. Talanta 100: 454–460.
Jayawardane, B. M., Wei, S., McKelvie, I. D., and Kolev, S. D. (2014a). Microfluidic paper–based analytical device for the determination of nitrite and nitrate. Analytical Chemistry 86: 7274–7279.
Jayawardane, B. M., Wongwilai, W., Grudpan, K., Kolev, S. D., Heaven, M. W., Nash, D. M., and McKelvie, I. D. (2014b). Evaluation and application of a paper–based device for the determination of reactive phosphate in soil solution. Journal of Environmental Quality. 43(3): 1081–1085.
Kalt, W., Forney, C. F., Martin, A., and Prior, R. L. (1999). Antioxidant capacity, vitamin C, phenolics, and anthocyanins after fresh storage of small fruits. Journal of Agricultural and Food Chemistry 47(11): 4638–4644.
Keller, M., and Hrazdina, G. (1998). Interaction of nitrogen availability during bloom and light intensity during veraison. II. Effects on anthocyanin and phenolic development during grape ripening. American Journal of Enology and Viticulture 49(3): 341–349.
Khaodee, W., Aeungmaitrepirom, W., and Tuntulani, T. (2014). Effectively simultaneous naked–eye detection of Cu (II), Pb (II), Al (III) and Fe (III) using cyanidin extracted from red cabbage as chelating agent. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 126: 98–104.
Koesdjojo, M. T., Pengpumkiat, S., Wu, Y., Boonloed, A., Huynh, D., Remcho, T. P., and Remcho, V. T. (2015). Cost effective paper–based colorimetric microfluidic devices and mobile phone camera readers for the classroom. Journal of Chemical Education 92(4): 737–741.
Landi, M., Tattini, M., and Gould, K. S. (2015). Multiple functional roles of anthocyanins in plant–environment interactions. Environmental and Experimental Botany 119: 4–17.
Lister, C. E., Lancaster, J. E., and Walker, J. R. (1996). Phenylalanine ammonia–lyase (PAL) activity and its relationship to anthocyanin and flavonoid levels in New Zealand–grown apple cultivars. Journal of the American Society for Horticultural Science 121(2): 281–285.
Mazza, G., and Brouillard, R. (1987). Recent developments in the stabilization of anthocyanins in food products. Food Chemistry 25(3): 207–225.
Meelapsom, R., Jarujamrus, P., Amatatongchai, M., Chairam, S., Kulsing, C., and Shen, W. (2016). Chromatic analysis by monitoring unmodified silver nanoparticles reduction on double layer microfluidic paper–based analytical devices for selective and sensitive determination of mercury(II). Talanta 155: 193-201.
Ministry of Public Health. (2002). Water Quality Standard. Retrieved from http://plan.dgr.go.th/school/5.pdf, December 4, 2019. (in Thai)
Mitchell–Koch, J. T., Reid, K. R., and Meyer-hoff, M.E. (2008). Salicylate detection by complexation with iron(III) and optical absorbance spectroscopy. Journal of Chemical Education 85(12): 1658–1659.
Nordberg, G., Fowler, B. and Nordberg, M. (2014). Handbook on the toxicology of metals. 4th ed. Amsterdam: Elsevier. Chapter 41, Iron. pp 879–902. Retrieved from http://dx.doi.org/10.1016/B978-0-444-59453-2.00041, April 28, 2020.
Perämäki, P., Kumpumäki, M., Välimäki, I., and Heikkinen, R. (2000). Preliminary studies of iron speciation (Fe2+ and Fe3+) in peat samples using polarography. Analytical Sciences 16: 751–756.
Pascual–Teresa, S., and Sanchez–Ballesta, T. (2008). Anthocyanins: From plant to health. Phytochemistry Reviews 7(2): 281–299.
Place, B. J. (2019). Activity analysis of iron in water using a simple led spectrophotometer. Journal of Chemical Education 96(4): 714–719.
Puchum, S., Meelapsom, R., Muniandy, S. S., Lee, H. L., Pencharee, S., Amatatongchai, M., Suttisintong, K., and Jarujamrus, P. (2019). Use of unmodified silver nanoparticles (AgNPs) as colorimetric Hg(II) sensor: A new approach to sensitive and high sample throughput determination of Hg(II) under high influence of ionic suppression. International Journal of Environmental Analytical Chemistry 99(2): 139–156.
Rapisarda, P., Tomaino, A., lo Cascio, R., Bonina, B., de Pasquale A., and Saija, A. (1999). Antioxidant effectiveness as influenced by phenolic content of fresh orange juices. Journal of Agricultural and Food Chemistry 47: 4718.
Rattanakaroonjit, W., Jarujamrus, P. and Su-pasorn, S. (2019). Development of conceptual understanding of oxidation–reduction reaction and determination of iron ions using smartphone-assisted colorimetric experiment. Proceedings of the Universal Academic Cluster Interna-tional Autumn Conferences (pp. 45–56). Bangkok, Thailand.
Sawyer, D. T., Heineman, W. R., and Beebe, J. M. (1984). Chemical Experiments for Instrumental Methods. New York: Wiley.
Schwarzenbach, R. P., Egli, T., Hofstetter, T. B., Gunten, U. V., and Wehrli, B. (2010). Global water pollution and human health. Annual Review of Environment and Re-sources 35: 109–136.
Sharma, G. (2002). Digital Color Imaging Handbook: Color Fundamentals for Digital Imaging. New York: CRC.
Sigurdson, G. T., and Giusti, M. M. (2014). Bathochromic and hyperchromic effects of aluminum salt complexation by anthocyanins from edible sources for blue color development. Journal of Agricultural and Food Chemistry 62(29): 6955–6965.
Sigurdson, G. T., Robbins, R. J., Collins, T. M., and Giusti, M. M. (2016). Evaluating the role of metal ions in the bathochromic and hyperchromic responses of cyanidin derivatives in acidic and alkaline pH. Food Chemistry 208: 26–34.
Silva, M. M., Vale M. G. R., Damin, I. C. F., Welz, B., Mandaji, M., and Fett, J. P. (2003). Method development for the determination of iron in milligram amounts of rice plants (Oryza sativa L.) from cultivation experiments using graphite furnace atomic absorption spectrometry. Analytical & Bioanalytical Chemistry 377: 165–172.
Skoog, D. A., West, D. M., Holler, F. J., and Crouch, S. R. (2013). Fundamental of Analytical Chemistry. 9th ed. Philadelphia: Cengage Learning.
Skoog, D. A., and Leary, J. J. (1992). Principles of Instrumental Analysis. 4th ed. Philadelphia: Saunders College.
Suziki, Y., Endo, M., Jin, J., Iwase, K., and Iwatsuki, M. (2006). Tristimulus colorimetry using a digital still camera and its application to determination of iron and residual chlorine in water samples. Analytical Sciences 22: 411–414.
Tachibana, N., Kimura, Y., and Ohno, T. (2014). Examination of molecular mechanism for the enhanced thermal stability of anthocyanins by metal cations and polysaccharides. Food Chemistry 143: 452–458.
Teaumroong, N., and Boonkerd, N. (1996). Iron element, siderophores and microbes. Suranaree Journal Science Technology 3: 95–100.
Trandafir, I., Nour, V., and Ionica, M. E. (2011). Determination of tin in canned foods by inductively coupled plasma–mass spectrometry. Polish Journal of Environmental Studies 21: 741–754.
Wattanayon, R., Satapor, S., Yama, B., Samanman, S., and Vangsirigul, P. (2017). Determination of iron ion using antho-cyanin from roselle. Princess of Naradhiwas University Journal 9(2): 97–103. (in Thai)
World Health Organization & International Programme on Chemical Safety. (1996). Guidelines for Drinking–Water Quality. Vol. 2, Health Criteria and Other Sup-porting Information. 2nd ed. World Health Organization. Retrieved from https://apps.who.int/iris/handle/10665/38551, December 4, 2019.
Xie, Y., Zhu, X., Li, Y., and Wang, C. (2018). Analysis of the pH–dependent Fe(III) ion chelating activity of anthocyanin extracted from black soybean [Glycine max (L.) Merr.] coats. Journal of Agricultural and Food Chemistry 66: 1131–1139.
Zhang, M., Zheng, B., Yuan, H., and Xiao, D. (2010). A spectrofluorimetric sensor based on grape skin tissue for determination of iron(III). Bulletin of the Chemical Society of Ethiopia 24(1): 31–37.