สปีชีส์ยีสต์ที่พบในลูกแป้งสาโทจากจังหวัดสุรินทร์: ความสามารถในการย่อยแป้งและการผลิตแอลกอฮอล์
Main Article Content
Abstract
Sasithorn Lorroengsil, Thanongsak Praseartsin, Nara Rodboonrit, Nareerat Moonjai and Jidapa Sangswan
รับบทความ: 29 เมษายน 2565; แก้ไขบทความ: 18 กันยายน 2565; ยอมรับตีพิมพ์: 23 กันยายน 2565; ตีพิมพ์ออนไลน์: 22 ธันวาคม
บทคัดย่อ
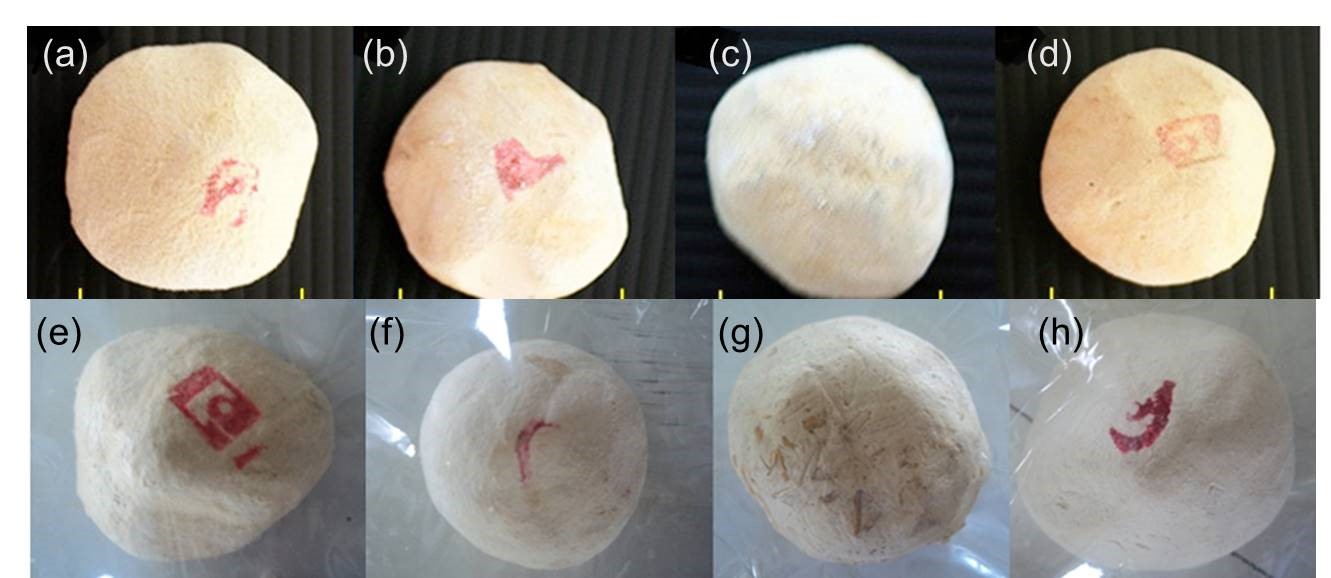
งานวิจัยนี้มีวัตถุประสงค์เพื่อคัดแยกเชื้อยีสต์จากลูกแป้งสาโทใน 6 อำเภอของจังหวัดสุรินทร์ ได้แก่ เมืองสุรินทร์ ปราสาท สำโรงทาบ ศรีขรภูมิ ท่าตูมและรัตนบุรี โดยนำตัวอย่างลูกแป้งที่ใช้ในการแยกยีสต์ จำนวน 8 ตัวอย่าง มาเพาะเชื้อในอาหารเหลว YM ที่เติม chloramphenicol ในอัตราส่วน 20 ppm และ sodium propionate 0.05% (น้ำหนัก/ปริมาตร) บ่มที่อุณหภูมิ 35 องศาเซลเซียส เป็นเวลา 24 ชั่วโมง จากนั้นนำมาขีดลากแบบไขว้ (cross streak) บนอาหารแข็ง YM บ่มที่อุณหภูมิ 35 องศาเซลเซียส เป็นเวลา 24–48 ชั่วโมง สามารถแยกยีสต์ได้ทั้งหมด 24 ไอโซเลต เมื่อจัดจำแนกโดยการเทียบลำดับเบสของบริเวณ D1/D2 ของ 26S rDNA ยีสต์จำนวน 20 ไอโซเลต มีผลการระบุชนิดของยีสต์เป็นยีสต์ 4 สปีชีส์ คือ Saccharomycopsis fibuligera (10 ไอโซเลต) Issatchenkia orientalis (5 ไอโซเลต) Pichia kudriavzevii (4 ไอโซเลต) และ Meyerozyma caribbica (1 ไอโซเลต) ซึ่งยีสต์ Sm. fibuligera เป็นสปีชีส์ที่พบบ่อยที่สุด โดยพบในลูกแป้งจำนวน 4 ตัวอย่าง จากทั้งหมด 8 ตัวอย่าง ลูกแป้งส่วนใหญ่พบยีสต์เพียงสปีชีส์เดียวยกเว้น 3 ตัวอย่างที่พบยีสต์ 2 สปีชีส์ โดยลูกแป้งจากอำเภอรัตนบุรีพบยีสต์ Sm. fibuligera ร่วมกับ P. kudriavzeii ลูกแป้งจากอำเภอศรีขรภูมิ (แสงจันทร์) พบยีสต์ Sm. fibuligera ร่วมกับ M. caribbica และลูกแป้งจากอำเภอปราสาท พบยีสต์ I. orientalis ร่วมกับ P. kudriavzevii เมื่อนำยีสต์ทั้ง 24 ไอโซเลตมาทดสอบความสามารถในการย่อยแป้ง พบว่ามียีสต์จำนวน 10 ไอโซเลตที่มีการเจริญและสร้างวงใสบนอาหาร starch agar ได้แก่ ไอโซเลต RTN1–1, RTN1–2, RTN1–3, SRT1–1, SRT1–2, SRT1–3, SRT1–4, SJ1–3, SJ1–4 และ TT1–2 ยีสต์ทั้ง-หมดมีขนาดวงใสใกล้เคียงกันยกเว้น RTN1-1 และ RTN1-3 ที่มีขนาดวงใสเล็กกว่าอย่างมีนัยสำคัญ อย่างไรก็ตามยีสต์จำนวน 10 ไอโซเลต ถูกระบุสปีชีส์เป็นยีสต์ Sm. fibuligera ส่วนความสามารถในการผลิตแอลกอฮอล์ในอาหารเลี้ยงเชื้อ fermentation broth ที่มีน้ำตาลซูโครส 10% (น้ำหนัก/ปริมาตร) พบว่ายีสต์ที่มีประสิทธิภาพในการผลิตแอลกอฮอล์สูงสุด คือ I. orientalis MSR2–2, MSR2–3 และ PS1–5 ซึ่งสามารถผลิตแอลกอฮอล์ได้ 5% (ปริมาตร/ปริมาตร) คิดเป็น 0.39 กรัมเอทานอล/กรัมซูโครส
คำสำคัญ: ยีสต์ ลูกแป้ง สุรินทร์ สาโท
Abstract
The objective of this research was to isolate yeast from the starter (loog–pang) of rice wine (sato) in 6 districts of Surin province including Mueang–Surin, Prasat, Samrong Thap, Sikhoraphum, Thatum and Rattanaburi. Eight samples of loog–pang were used to isolate yeast by adding to YM broth supplemented with 20 ppm of chloramphenicol and 0.05%(w/v) sodium propionate. The flasks were incubated at 35°C for 24 hours. Then yeasts in cultured medium were isolated on YM agar using the cross–streaking method after incubation at 35°C for 24–48 hours. Twenty–four of yeast isolates were obtained in present study. The yeast identification procedure based on the sequencing of amplified D1/D2 region of the yeast 26S ribosomal DNA, twenty of yeast isolates were identified to 4 species including Saccharomycopsis fibuligera (10 isolates), Issatchenkia orientalis (5 isolates), Pichia kudriavzevii (4 isolates) and Meyerozyma caribbica (1 isolate). Yeast Sm. fibuligera was found in 4 samples from total 8 samples which was the most frequently found species in loog-pang. Only one yeast species was found mostly in loog–pang samples, except for 3 samples which two yeast species were found. Yeasts Sm. fibuligera and P. kudriavzeii were found in loog–pang samples from Rattanaburi district, Sm. fibuligera and M. caribbica were found in loog–pang samples from Sikhoraphum (Sang–Jan) district and I. orientalis and P. kudriavzevii were found in loog–pang samples from Prasat district. Among 24 isolates which were tested for starch hydrolysis, it was found that 10 isolates (RTN1–1, RTN1–2, RTN1–3, SRT1–1, SRT1–2, SRT1–3, SRT1–4, SJ1–3, SJ1–4 and TT1–2) could grow and produce clear zone on starch agar. The clear zone diameters of all isolates were similar, except for isolate RTN1–1 and RTN1–3 which were significantly smaller than others. However, all 10 isolates were identified species as Sm. fibuligera. Alcohol production was examined in fermentation broth containing 10%(w/v) sucrose. The highest alcohol contents of 5.0%(v/v) were obtained from I. orientalis isolates MSR2–2, MSR2–3 and PS1–5 with ethanol yield of 0.39 gethanol/gsucrose.
Keywords: Yeast, Loog–pang, Surin, Sato
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
References
Chaijamrus, S., and Mouthung, B. (2011). Selection of Thai starter components for ethanol production utilizing malted rice from waste paddy. Songklanakarin Journal of Science and Technology 33(2): 163–170.
Chamnipa, N., Thanonkeo, S., Klanrit, P., and Thanonkeo, P. (2018). The potential of the newly isolated thermotolerant yeast Pichia kudriavzevii RZ8–1 for high–temperature ethanol production. Brazilian Journal of Microbiology 49(2): 378–391.
Chanchaichaovivat, A., Phornphisutthimas, S., and Mookseang, K. (2015). Analysis and comparison of yeast β–glucan from Loog–Pang Kao–Mak in the central part of Thailand. Journal of Research Unit on Science, Technology and Environment for Learning 6(2): 188–197. (in Thai)
Cheenacharoen, S., and Juntachai, W. (2018). Diversity and genetic relationship of ethanol tolerant yeasts isolated from rice wine starters (Loog–Pang) in Chiang Mai province, Thailand. Journal of Science and Technology 26(3): 478–489. (in Thai)
Chomchoei, A. (2005). Screening of Microbial in Lookpang for Pure–inoculum Development in Rice Fermented Products. Thesis of Chiang Mai Rajabhat University, Chiang Mai (Thailand). Aggie Technology Faculty; Pasu Pramokchon. (in Thai)
Daniel, H. M., Vrancken, G., Takrama, J. F., Camu, N., de Vos, P., and de Vuyst, L. (2009). Yeast diversity of Ghanaian cocoa bean heap fermentations. FEMS Yeast Research 9: 774–783.
Daroonpunt, R., Tanasupawat, S., and Keeratipibul, S. (2016). Characterization and amylolytic activity of yeast and mold strains from Thai sweet rice. Malaysian Journal of Microbiology 12(2): 121–131.
Dhaliwal, S., Oberoi, H., Sandhu, S., Nanda, D., Kumar, D., and Uppal, S. (2011). Enhanced ethanol production from sugarcane juice by galactose adaptation of a newly isolated thermotolerant strain of Pichia kudriavzevii. Bioresource Technology 102: 5968–5975.
Farh, M. E.–A., Cho, Y., Lim, J. Y., and Seo, J.–A. (2017). A diversity study of Saccharomycopsis fibuligera in rice wine starter nuruk, reveals the evolutionary process associated with its interspecies hybrid. Journal of Microbiology 55: 337–343.
Guo, Z.–P., Zhang, L, Ding, Z.–Y., Gu Z.–H., and Shi, G.–Y. (2011). Development of an industrial ethanol–producing yeast strain for efficient utilization of cellobiose. En-zyme and Microbial Technology 49(1): 105–112.
Hisamatsu, M., Furubayashi, T., Karita, S., Mishima, T., and Isono, N. (2006). Isolation and identification of a Novel yeast fermenting ethanol under acidic conditions. Journal of Applied Glycoscience 53(2): 111–113.
Isono, N., Hayakawa, H., Usami, A., Mishima, T., and Hisamatsu, M. (2012). A comparative study of ethanol production by Is-satchenkia orientalis strains under stress conditions. Journal of Bioscience and Bio-engineering 113: 76–78.
Jung, M. J., Nam, Y. D., Roh, S. W., and Bae, J.W. (2012). Unexpected convergence of fungal and bacterial communities during fermentation of traditional Korean alcoholic beverages inoculated with various natural starters. Food Microbiology 30: 112–123.
Kaewkongpan, D., Chompubai, P., Punyapliew, S., and Punaui, O. (2017). A comparative study of the qualities of fruit Sato brewed from a variety of ingredients. Scientific Research Journal Lampang Rajabhat University 2(1): 17–28. (in Thai)
Kitagawa, T., Tokuhiro, K., Sugiyama, H., Kohda, K., Isono, N., Hisamatsu, M., Takahashi, H., and Imaeda, T. (2010). Construc-tion of a β–glucosidase expression system using the multistress–tolerant yeast Issatchenkia orientalis. Applied Microbiology and Biotechnology 87: 1841–1853.
Koh, J. H., and Suh, H. J. (2009). Biological activities of thermotolerant microbes from fermented rice bran as an alternative micro-bial feed additive. Applied Biochemistry and Biotechnology 157: 420–430.
Kotaka, A., Bando, H., Kaya, M., Kato–Murai, M., Kuroda, K., Sahara, H., Hata, Y., Kondo, A., and Ueda, M. (2008). Direct ethanol production from barley β–glucan by sake yeast displaying Aspergillus oryzae β–glucosidase and endoglucanase. Journal of Bioscience and Bioengineering 105(6): 622–627.
Kunlabut. A., Porntrai, S., and Sangswan, J. (2020). Ethanol production from Loog–Pang–Sato yeast cultivation with molasses. Journal of Research Unit on Science, Technology and Environment for Learn-ing 11(1): 53–65. (in Thai)
Kwon, Y. J., Ma, A. Z., Li, Q., Wang, F., Zhuang, G. Q., and Liu, C. Z. (2011). Effect of lignocellulosic inhibitory compounds on growth and ethanol fermentation of newly–isolated thermotolerant Issatchenkia orientalis. Bioresource Technology 102(17): 8099–8104.
Limtong, S., Sintara, S., Suwanarit, P., and Lotong, N. (2005). Species diversity of molds in Thai traditional fermentation starters (Loog–Pang). Kasetsart Journal (Natural Science) 39: 511–518.
Limtong, S., Sintara, S., Suwannarit, P., and Lotong, N. (2002). Yeast diversity in Thai traditional alcoholic starter (Loog–pang). Kasetsart Journal (Natural Sciences) 36(2): 149–158.
Luangkhlaypho, A., Pattaragulwanit, K., Leepipatpiboon, N., and Yompakdee, C. (2014). Development of a defined starter culture mixture for the fermentation of sato, a Thai rice–based alcoholic beverage. Science Asia 40(2): 125–134.
Lv, X. C., Huang, X. L., Zhang, W., Rao, P. F., and Ni, L. (2013). Yeast diversity of tradi-tional alcohol fermentation starters for Hong Qu glutinous rice wine brewing, revealed by culture–dependent and culture–independent methods. Food Control 34: 183–190.
Nonklang, S. (2012). Factor affecting the trans-formation efficiency of linear DNA in yeast Kluyveromyces marxianus UBU–1–1. Jour-nal of Science and Technology, Ubon Ratchathani University 14(4): 34–41.
Nuanpeng, S. (2018). Comparison rice vinegar production from Hom–nil rice and Riceberry rice. Agricultural Science Journal 49(2): 605–608.
Oberoi, H. S., Babbar, N., Sandhu, S. K., Dha-liwal, S. S., Kaur, U., Chadha, B. S., and Bhargav, V. K. (2012). Ethanol production from alkali–treated rice straw via simultaneous saccharification and fermentation using newly isolated thermotolerant Pichia kudriavzevii HOP–1. Journal of Indus-trial Microbiology and Biotechnology 39(4): 557–566.
Paewlueng, P., Deeseenthum, S., and Rattanasuk, S. (2019). Screening of yeasts from Thai traditional fermentation starter (Loog–pang) for alcoholic fermentation products in community enterprise. ICoFAB2019 Proceedings (pp. 74–78). Doi: 10.14457/MSU.res.2019.15.
Patsalou, M., Samanides, C., Protopapa, E., Stavrinou, S., Vyrides, I., and Koutinas, M. (2019). A citrus peel waste biorefinery for ethanol and methane production. Molecules 24: 2451.
Prillinger, H., Molnár, O., Eliskases–Lechner, F., and Lopandic, K. (1999). Phenotypic and genotypic identification of yeasts from cheese. Antonie Van Leeuwenhoek 75: 267–283.
Rakmai, J., Cheirsilp, B., and Srinuanpan, S. (2019). Designation of rice cake starters for fermented rice products with desired characteristics and fast fermentation. Journal of Food Science and Technology 56(6): 3014–3022.
Roongrojmongkhon, N., Rungjindamai, N., Vata-navicharn, T., and Ochaikul, D. (2020). Isolation and identification of fungi with glucoamylase activity from Loog–pang–khao–mak (A Thai traditional fermentation starter). Journal of Pure and Applied Microbiology 14: 233–247.
Sandhu, S., Oberoi, H., Dhaliwal, S., Babbar, N., Nanda, D. and Kumar, D. (2011). Ethanol production from Kinnow mandarin (Citrus reticulata) peels via simultaneous saccharification and fermentation using crude enzyme produced by Aspergillus oryzae and the thermotolerant Pichia ku-driavzevii strain. Annals of Microbiology 62: 1–12.
Steinkraus, K. H. (1997). Classification of fermented foods: Worldwide review of house-hold fermentation techniques. Food Control 8: 331–317.
Sukpipat, W., Komeda, H., Prasertsan, P., and Asano, Y. (2017). Purification and characterization of xylitol dehydrogenase with l–arabitol dehydrogenase activity from the newly isolated pentose–fermenting yeast Meyerozyma caribbica 5XY2. Journal of Bioscience and Bioengineering 123(1): 20–27.
Supcharoenlert, A. (2010). Isolation of Molds and Yeasts from Lookpang. Master of Engineering (Chemical Engineering). Bangkok: King Mongkut's University of Technology Thonburi.
Tachaapikoon, C., Rattanapatpokin, T., Waeonukul, R., Wattanachaisaereekul, S., Ratanakhanokchai, K., and Pason, P. (2019). Newly isolated malic acid fermenting yeast Meyerozyma caribbica AY 33–1 for bioconversion of glucose and cassava pulp. SEATUC Journal of Science and Engineering 1: 62–70.
Taechavasonyoo, A., Thaniyavarn, J., and Yompakdee, C. (2013). Identification of the molds and yeasts characteristic of a superior Loogpang, starter of Thai rice based alcoholic beverage Sato. Asian Journal of Food and Agro–Industry 6: 24–38.
Tamang, J. P., and Thapa, S. (2006). Fer-mentation dynamics during production of bhaati jaanr, a traditional fermented rice beverage of the Eastern Himalayas. Food Biotechnology 20: 251–261.
Tamang, J. P., Watanabe, K., and Holzapfel, W. H. (2016). Review: Diversity of microorganisms in global fermented foods and beverages. Frontiers in Microbiology 7: 377.
Thalagala, T. A. T. P., Kodama, S., Mishima, T., Isono, N., Furujyo, A., Kawasaki, Y., and Hisamatsu, M. (2009). Study on ethanol fermentation using D–glucose rich frac-tions obtained from lignocelluloses by a two–step extraction with sulfuric acid and Issatchenkia orientalis MF121. Journal of Applied Glycoscience 56: 7–11.
Trongjit, K., and Suwannapusit, U. (2020). Fermented food technology, local wisdom and eating culture of ethnic groups along the Thailand – Cambodia Border regions. Koch Cha Sarn Journal of Science 42(2): 23–39. (in Thai)
Yuan, S.–F., Guo, G.–L., and Hwang, W.–S. (2017). Ethanol production from dilute–acid steam exploded lignocellulosic feed-stocks using an isolated multistress–tole-rant Pichia kudriavzevii strain. Microbial Biotechnology 10(6): 1581–1590.
Yuangsaard, N., Yongmanitchai, W., Yamada, M., and Limtong, S. (2013). Selection and characterization of a newly isolated ther-motolerant Pichia kudriavzevii strain for ethanol production at high temperature from cassava starch hydrolysate. Antonie van Leeuwenhoek 103: 577–588.