การคัดเลือกบาซิลลัสที่ผลิตแบคเทอริโอซินต้านเชื้อก่อโรคปลาจากอาหารหมักและการศึกษาคุณสมบัติเบื้องต้นของแบคเทอริโอซิน
Main Article Content
Abstract
Siriporn Tipsing, Saowanit Tongpim and Ratchanu Meidong
รับบทความ: 27 พฤศจิกายน 2561; แก้ไขบทความ: 23 มีนาคม 2562; ยอมรับตีพิมพ์: 30 เมษายน 2562
บทคัดย่อ
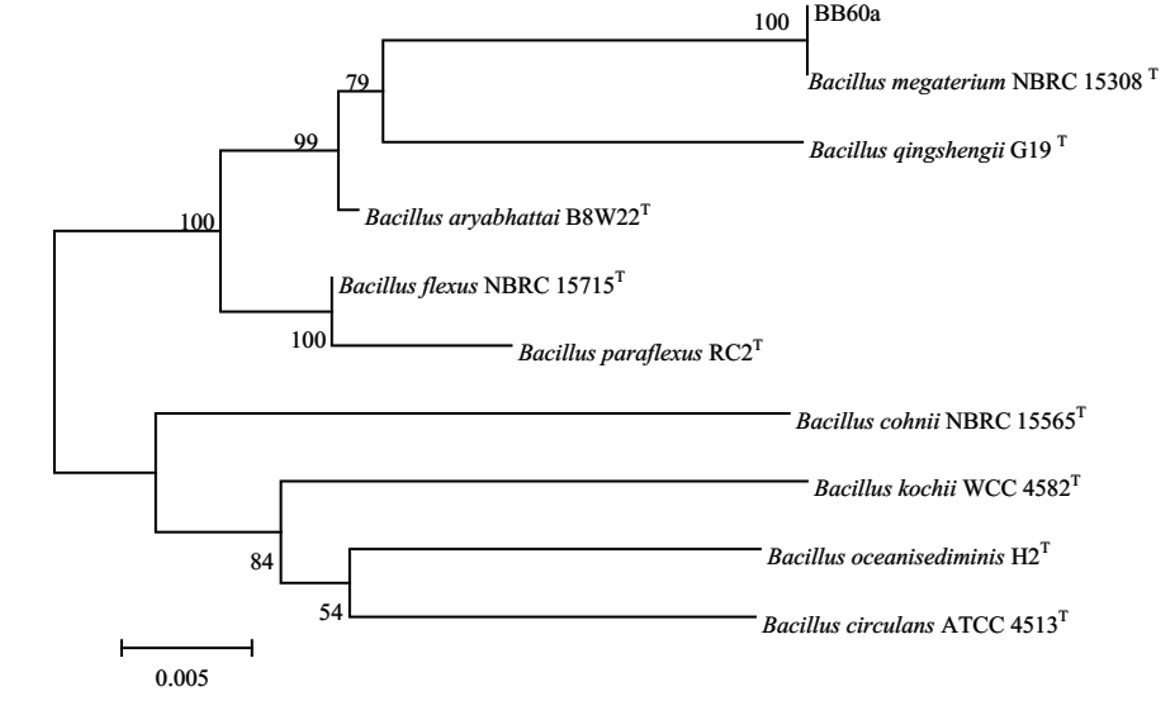
งานวิจัยนี้มีวัตถุประสงค์เพื่อคัดเลือกและศึกษาคุณสมบัติของแบคเทอริโอซินจากบาซิลลัสที่แยกจากอาหารหมักของไทย โดยบาซิลลัส 258 ไอโซเลตแยกมาจากตัวอย่างอาหารหมักจำนวน 62 ตัวอย่างและนำไปศึกษาความสามารถในการผลิตแบคเทอริโอซินต้านเชื้อก่อโรคปลาด้วยวิธี agar well diffusion assay ผลการวิจัยพบว่า ไอโซเลต BB60a ซึ่งแยกมาจากผลิตภัณฑ์ปลาส้ม มีประสิทธิภาพสูงสุดในการยับยั้งเชื้อก่อโรคปลาที่เป็นแบคทีเรียแกรมบวก (Streptococcus agalactiae) และแบคทีเรียแกรมลบ (Aeromonas hydrophila) เมื่อจำแนกระบุชนิดของไอโซเลต BB60a ด้วยลักษณะทางสัณฐานวิทยาร่วมกับลำดับ 16S rDNA พบว่า มีความใกล้เคียงกับ Bacillus megaterium ร้อยละ 99.83 นอกจากนี้ยังศึกษาผลของแหล่งไนโตรเจนต่อ B. megaterium BB60a ในการผลิตสารแบคเทอริโอซิน พบว่า yeast extract ความเข้มข้น 12 กรัมต่อลิตรที่เป็นองค์ประกอบของอาหารเลี้ยงเชื้อช่วยส่งเสริมให้เชื้อผลิตแบคเทอริโอซินได้สูงสุด (p < 0.05) จากการศึกษาคุณสมบัติเบื้องต้นของสารแบคเทอริโอซินพบว่ามีความเสถียรในช่วง pH 4–10 และในช่วงอุณหภูมิ 40–70ºC และพบ ว่าสารนี้ถูกทำลายได้ด้วยเอนไซม์ trypsin และ proteinase K แต่ทนต่อเอนไซม์ α-amylase และ lipase จากผลการศึกษานี้สรุปได้ว่าแบคเทอริโอซินจาก B. megatherium BB60a ออกฤทธิ์การยับยั้งเป็นแบบ broad spectrum ทนสภาพกรด–เบสได้ในช่วงกว้าง และทนต่อความร้อน จึงเหมาะสมที่จะนำไปประยุกต์ใช้ในการเลี้ยงปลาเพื่อยับยั้งการติดเชื้อก่อโรคปลาคำสำคัญ: บาซิลลัส แบคเทอริโอซิน เชื้อก่อโรคปลา
Abstract
The aims of this study were to screen and preliminarily characterize the properties of bacteriocin produced by Bacillus isolated from Thai fermented foods. In this study, 258 isolates of Bacillus were obtained from 62 samples of Thai fermented foods and screened for their bacteriocin against fish pathogens. Antimicrobial activity of bacteriocin was evaluated using an agar well diffusion assay. Isolate BB60a derived from fermented fish product showed the highest antimicrobial activities against fish pathogens which were both Gram positive (Streptococcus agalactiae) and Gram negative (Aeromonas hydrophila) bacteria. Bacterial identification based on cell morphology and 16S rDNA sequence revealed that the isolate BB60a was closely related to Bacillus megaterium (99.83% similarity). The effect of nitrogen sources on bacteriocin production was investigated. B. megaterium BB60a produced the highest bacteriocin when cultivated in the medium containing 12 g/L yeast extract. This bacteriocin showed stability over a wide range of pH (4–10) and temperatures (40–70ºC). It was denatured completely by trypsin and proteinase K, but not by α-amylase and lipase. From this study, it can be concluded that B. megaterium BB60a produces a broad-spectrum bacteriocin that tolerates acidic, alkaline and high temperature conditions and, therefore, it has the potential application in fish culture to prevent bacterial fish infection.
Keywords: Bacillus, Bacteriocin, Fish pathogens
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
References
Anthony, T., Rajesh, T., Kayalvizhi, N., and Gunasekaran, P. (2009). Influence of medium components and fermentation conditions on the production of bacteriocin(s) by Bacillus licheniformis AnBa9. Bioresource Technology 100(2): 872–877.
Ayed, H. Ben, Maalej, H., Hmidet, N., and Nasri, M. (2015). Isolation and biochemical characterisation of a bacteriocin–like substance produced by Bacillus amyloliquefaciens An6. Journal of Global Antimicrobial Resistance 3(4): 255–261.
Cherif, A., Rezgui, W., Raddadi, N., Daffonchio, D., and Boudabous, A. (2008). Characterization and partial purification of entomocin 110, a newly identified bacteriocin from Bacillus thuringiensis subsp. entomocidus HD110. Microbiological Research 163: 684–692.
Cladera-Olivera, F., Caron, G. R., and Brandelli, A. (2004). Bacteriocin–like substance pro-duction by Bacillus licheniformis strain P40. Letters in Applied Microbiology 38: 251–256.
Das, S., Ward, L. R., and Burke, C. (2010). Screening of marine Streptomyces spp. for potential use as probiotics in aqua-culture. Aquaculture 305: 32–41.
Dobson, A., Cotter, P. D., Ross, R. P. and Hill, C. (2012). Bacteriocin production: a probiotic trait? Applied and Environmen-tal Microbiology 78(1): 1–6.
Embaby, A. M., Heshmat, Y., Hussein, A., Marey, H. S. (2014). A sequential statistical approach towards an optimized production of a broad spectrum bacteriocin substance from a soil bacterium Bacillus sp. YAS 1 strain. The Scientific World Journal vol. 2014, Article ID 396304.
FAO/WHO. (2006) Probiotics in food. Health and nutritional properties and guidelines for evaluation. FAO Food and Nutrition Paper: 85.
Fishery Information Technology Center. (2017). Fisheries statistics of Thailand 2015. Technical paper No. 5/2017. Fishery Information Technology Center, Department of Fisheries, Ministry of Agriculture and Cooperatives: 9.
Fontana, L., Bermudez–Brito, M., Plaza–Diaz, J., Munoz–Quezada, S., and Gil, A. (2013). Sources, isolation, characterization and evaluation of probiotics. British Journal of Nutrition 109: Suppl, S35–S50.
Gobi, N., Vaseeharan, B., Chen, J., Rekha, R., Vijayakumar, S., Anjugam, M., and Iswarya, A. (2018). Fish and Shell fish Immunology Dietary supplementation of probiotic Bacillus licheniformis Dahb1 improves growth performance, mucus and serum immune parameters, antioxidant enzyme activity as well as resistance against Aeromonas hydrophila in tilapia Oreochromis mossambicus. Fish and Shellfish Immunology 74: 501–508.
Guo, X., Li, D., Lu, W., Piao, X., and Chen, X. (2006) Screening of Bacillus strains as potential probiotics and subsequent confirmation of the in vivo effectiveness of Bacillus subtilis MA139 in pigs. Antonie Van Leeuwenhoek 90: 139–146.
Hassaan, M. S., Soltan, M. A., Mohammady, E. Y., Elashry, M. A., El–haroun, E. R., and Davies, S. J. (2018). Growth and physiological responses of Nile tilapia, Oreochromis niloticus fed dietary fermented sun flower meal inoculated with Saccharomyces cerevisiae and Bacillus subtilis. Aquaculture 495(June): 592–601.
Hwanhlem, N., Ivanova, T., Biscola, V., Choi-set, Y., and Haertlé, T. (2017). Bacteriocin producing Enterococcus faecalis isolated from chicken gastrointestinal tract originating from Phitsanulok, Thailand: Isolation, screening, safety evaluation and probiotic properties. Food Control 78: 187–195.
Inatsu, Y., Kimura, K., and Itoh, Y. (2002). Characterization of Bacillus subtilis strains isolated from fermented soybean foods in Southeast Asia: Comparison with B. subtilis (natto) starter strains. Japan Agricultural Research Quarterly 36: 169–175.
Kim, O., Cho, Y. J., Lee, K., Yoon, S. H., Kim, M., Na, H., Park, S. C., Jeon, Y. S., Lee, J. H., Yi, H., Won, S., and Chun, J. (2012). Introducing EzTaxon-e: A prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species, (2012). International Journal of Systematic and Evolutionary Microbiology 62: 716–721.
Lee, S. G., and Chang, H. C. (2018). Purification and characterization of mejucin, a new bacteriocin produced by Bacillus subtilis SN7. LWT – Food Science and Technology 87: 8–15.
Maisak, H., Jantrakajorn, S., Lukkana, M., and Wongtavatchai, J. (2013) Antibacterial activity of tannin from sweet chestnut wood against Aeromonas and Streptococcal pathogens of tilapia (Oreochromis niloticus). The Thai Journal of Veterinary Medicine 43: 105–111.
Meidong, R., Doolgindachbaporn, S., Jamjan, W., Sakai, K., Tashiro, Y., Okugawa, Y., and Tongpim, S. (2017). A novel probiotic Bacillus siamensis B44v isolated from Thai pickled vegetables for potential use as a feed supplement in aquaculture. The Journal of General and Applied Microbiology 253: 246–253.
Meidong, R., Khotchanalekha, K., Doolgindach-baporn, S., Nagasawa, T., Nakao, M., Sakai, K., and Tongpim, S. (2018). Evaluation of probiotic Bacillus aureus B81e isolated from healthy hybrid catfish on growth, disease resistance and innate immunity of Pla–mong Pangasius bocourti. Fish and Shellfish Immunology 73: 1–10.
Nayak, S. K. (2010). Probiotics and immunity: A fish perspective. Fish and Shellfish Immunology 29(1): 2–14.
Newaj–Fyzul, A., and Austin, B. (2015). Probiotics, immunostimulants, plant products and oral vaccines, and their role as feed supplements in the control of bacterial fish diseases. Journal of Fish Diseases 38(11): 937–955.
O'Sullivan, L., Ross, R. P., and Hill, C. (2002). Potential of bacteriocin-producing lactic acid bacteria for improvements in food safety and quality. Biochimie 84(5-6): 593–604.
Parente, E., Ricciardi, A., and Addario, G. (1994). Influence of pH on growth and bacteriocin production by Lactococcus lactis subsp. lactis 140NWC during batch fermentation. Applied Microbiology and Biotechnology 41: 388–394.
Regulation (EC), (2003) No 1831/2003 of the European parliament and of the council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10. 2003, p.29. Last amended by Regulation (EC) No 386/2009, OJ L 118, 13.5.2009, p.66.
Salazar, F., Ortiz, A., and Sansinenea, E. (2017). Characterization of two novel bacteriocin-like substances produced by Bacillus amyloliquefaciens ELI149 with broad–spectrum antimicrobial activity. Journal of Global Antimicrobial Resistance 11: 177–182.
Scholz, R., Vater, J., Budiharjo, A., Wang, Z., He, Y., and Dietel, K. (2014). Amylocyclicin, a novel circular bacteriocin produced by Bacillus amyloliquefaciens FZB42. Journal of Bacteriology 96: 1842–1852.
mith, P. (2008) Antimicrobial resistance in aquaculture. Revue Scientifique et Technique 27: 243–264.
Tapia–Paniagua, S. T., Vidal, S., Lobo, C., Prieto–Álamo, M. J., Jurado, J., Cordero, H., Cerezuelad, R., García de la Bandab, I., Esteband, M. A., Balebonaa, M. C., and Moriñigoa, M.A. (2014). The treatment with the probiotic Shewanella putrefaciens Pdp11 of specimens of Solea senegalensis exposed to high stocking densities to enhance their resistance to disease. Fish and Shellfish Immunology 41(2): 209–221.
Tongpim, S., Meidong, R., Nontaso, N., and Doolgindachbaporn, S. (2009) Screening of lactic acid bacteria to be used as probiotics in Tilapia fish. The 3th International Conference on Fermentation Technology for Value added Agricultural Products. Klangnanatham, Khon Kaen: 55.
Xia, Y., Lu, M., Chen, G., Cao, J., Gao, F., Wang, M., Liu, Z., Zhang, D., Zhu, H., and Yi, M. (2018). Effects of dietary Lactobacillus rhamnosus JCM1136 and Lactococcus lactis subsp. lactis JCM5805 on the growth, intestinal microbiota, morphology, immune response and disease resistance of juvenile Nile tilapia, Oreochromis niloticus. Fish and Shellfish Immunology 76: 368–379.