Determination of Fe3+ in Water Samples by Reduced Schiff Base Ligand
Keywords:
Reduced Schiff base ligand, Sensor, Fe3 , Water samplesAbstract
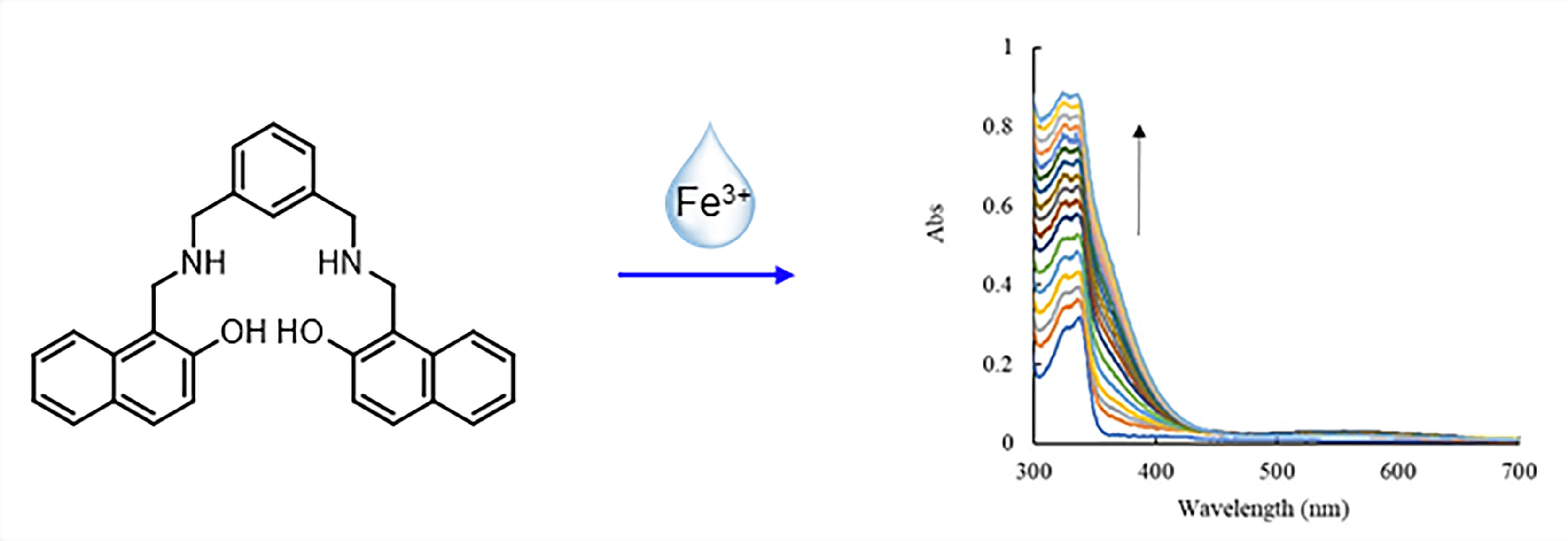
This research presents the development of a method for the quantification of Fe3+ using a reduced Schiff base ligand, which was synthesized via a simple two-step process with a high yield of 84%. The quantification of Fe3+ was analyzed using UV-Visible spectrophotometry. The selective binding of ligand with various cations (Fe3+, Cr3+, Pb2+, Co2+, Cu2+, Zn2+, Cd2+, Hg2+, Mn2+, Ni2+, Fe2+, Mg2+, Ag+, Na+, K+) was investigated. The results revealed that the reduced Schiff base ligand specifically formed a complex with Fe3+, indicated by a new absorption peak in the 350–400 nm range due to ligand-to-metal charge transfer (LMCT). In contrast, other cations induced no significant spectral changes. Quantitative studies of Fe3+ showed that the absorbance at 380 nm increased linearly with Fe3+ concentration, displaying a correlation coefficient (R2) of 0.9994 within the Fe3+ concentration range of 2.00 × 10–6 to 1.00 × 10–3 M. The method exhibited a limit of detection (LOD) of 1.22 × 10–6 M and a limit of quantification (LOQ) of 2.03 × 10–5 M. The reduced Schiff base ligand was successfully applied for Fe3+ quantification in real water samples, corresponding to the results obtained by inductively coupled plasma optical emission spectroscopy (ICP-OES).Downloads
Download data is not yet available.
Downloads
Published
2024-12-25
How to Cite
Doungchandeng, S., Arpiratikul, N., Sungwienwong, I. ., Sriwilai, P. ., & Thongruang, P. (2024). Determination of Fe3+ in Water Samples by Reduced Schiff Base Ligand . Science Essence Journal, 40(2), 12–25. Retrieved from https://ejournals.swu.ac.th/index.php/sej/article/view/16464
Issue
Section
Research Article







